Green Hydrogen Production
Design and Analysis High Efficiency Alkaline Electrolyzer
Scroll ↓
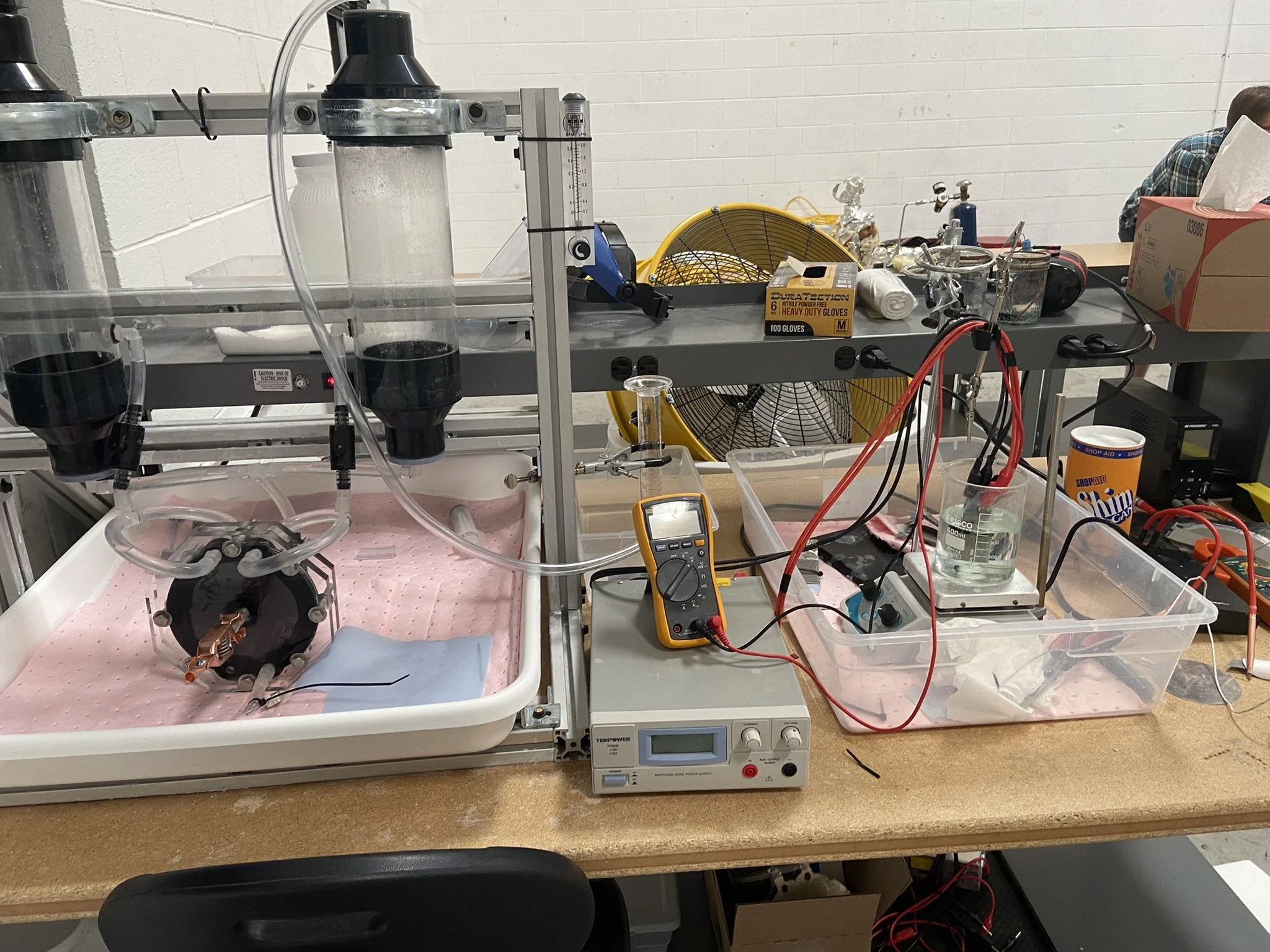
High Efficiency Alkaline Electrolyzer System - Dual Stacks
How Alkaline Electrolyzer Work?
An alkaline electrolyzer is a device used to produce hydrogen gas through the process of water electrolysis. This technique involves the use of electricity to split water (H₂O) into hydrogen (H₂) and oxygen (O₂) gases. Electrolyzers apply force (voltage) to change how atoms share electrons to create different compounds. In practice, when electrical current is applied to the electrolyzer, water molecules at the cathode gain electrons (reduction), forming hydrogen gas and hydroxide ions (OH⁻). At the anode, water molecules lose electrons (oxidation), forming oxygen gas and additional hydroxide ions. The overall reaction can be summarized as:
Cathode: 2H2O(l)+2e−→H2(g)+2OH−(aq)
Anode: 2OH−(aq)→0.5O2(g)+H2O(l)+2e−
Overall reaction: H2O(l)→H2(g)+0.5O2(g)
Alkaline Electrolizer Components
An alkaline electrolyzer is composed of several key components that work together to split water molecules into hydrogen and oxygen gases. The main components are:
(1) Electrodes: Typically made of materials like stainless steel (California Banned) or nickel, the electrodes are where the reduction and oxidation reactions occur. The cathode is the site of hydrogen gas production, and the anode is where oxygen gas is generated.
(2) Electrolyte: A concentrated solution of potassium hydroxide (KOH) or sodium hydroxide (NaOH) is used as the electrolyte. This alkaline solution facilitates ionic conduction between the electrodes.
(3) Separator: This component separates the anode and cathode compartments, preventing the mixing of hydrogen and oxygen gases while allowing the passage of hydroxide ions. Common materials for the diaphragm include asbestos, polysulfone matrix, and Zirfon.
(4) End Pressure Plates: These plates provide structural integrity and support to the electrolyzer, ensuring that the internal components remain in place and under the necessary pressure.
(5) Sealing Gaskets: These are used to prevent leaks of the electrolyte solution and gases, ensuring the safe and efficient operation of the electrolyzer.
(6) Current Collectors: Also known as gas diffusion layers, these components collect the current and facilitate the efficient flow of electrons through the system.
Assembly of an Alkaline Electrolyzer
Measuring Efficiency
Every electrochemical reaction requires a minimum amount of voltage to occur. Various sources of resistance increase the actual voltage needed to drive the reaction in real-world conditions. Efficiency is calculated by dividing the theoretical voltage by the total applied voltage. For water splitting, the theoretical minimum is 1.23 volts, so an electrolyzer operating at 1.75 volts would be approximately 70% efficient. Three primary sources of resistance are: Imperfect catalysts, and they require extra voltage to run the reaction at a reasonable rate. Long distance between electrodes, ions need force to push them between the electrodes, and the required force increases as current (electron flow) as distance increase. Ions face difficulty traversing membranes, diaphragms, or separators that separate the anode and the cathode
How my Design Solve These Problems?
1) Anode/Cathode Catalyst Overpotential
Nickel alloy powder treatment is applied to one side of the electrode, while the other side is made of porous nickel. This combination enhances the electrolysis surface area and allows the generated gas to move easily through the porous structure. My Lab experiments have shown that this approach increases efficiency by up to 12%.
2) Electrolyte Resistance
Reducing the gap between electrodes improves efficiency as the current has a shorter distance to travel. The Zero Gap Separator design addresses this issue by reducing the gap to 50 micrometers and eliminating traditional piping systems, thereby facilitating easier gas flow.
Traditional Cell vs. Zero Gap Cell
3)Separator Resistance
The membrane, a non-conductive polymer, is a crucial component in a zero gap cell. It separates gases, determines the purity of the gases, and allows the passage of current and ions. After testing and analyzing dozens of materials, I found that PES-50 is the best material for this membrane.
Nickle Powder Treatment (left), Porous Nickle (middle), PES-50 Membrane (right)
Production Cost
The cost to make one stack with 28 cell alkaline electrolyzer is 2400$ bring down the cost production as low as 3$/kg including electricity cost from solar panel. This is almost 1/3 of the market in 2024.
Render of Zero Gap Separator
Design Pressure: 0.1 barg
Design Temperature: 90C
Ambient Temperature: 10-45C
Hydrogen Production Cost: 3$/kg
Current Density: 1.2 A/cm2
Operation Voltage: 1.75V/cell
Electrical Efficiency: 70 %
Hydrogen Purity: > 99%
Oxygen Purity: > 99.5%
Electrolyte: KOH 30%
Full Stack of Zero Gap Cell Electrolyzer with 28-Cells
Lab Scale of the Zero Gap Cell Electrolyzer with 5-Cells